Introduction
Laser-cladding (LC) is an enhancement technology for surface finishing of metallic components; it involves covering the low-cost materials with a pore- and crack-free alloy to protect the component from wears and/or corrosions. Physically, LC is similar to traditional arc-welding but employing laser as the heating source that, in turn, widens the form of the overlay materials (i.e., powders, wires, strips, etc.) as well as their supplying method (i.e., prepositioning and/or continuously feeding). The laser heating can be controlled to form metallic bonds between the overlay alloy and the surface of the components with minimized substrate dilution while eliminate the distortion and performance degradation of the base material. The rapid heating and cooling of LC tend to increase the solid solubility and reduce the grain size of the resultant metastable alloys, offering important applications in developing novel alloys with advanced functions.
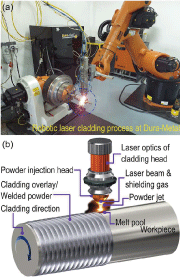
Recent developments in digital control and industrial robotic platform (IRP) are pushing the digitalization of the LC process at an unprecedented pace [1]. Figure 1 shows an example, i.e., a typical LC integrated on an IRP at Dura-Metal (a Singapore manufacturer) for protective coatings. Technically, the powder feeding can be switched among multiple sources and the cladding can thus be monolithically conducted with gradually varied compositions. This capability, combined with recent developed AI-based approaches, tremendously accelerates the material developments for particular applications.
Here, LC process of inconel 625 (In625) and stainless steel (SS431) is conducted on low alloy steel (LAS) substrates (AISI 4140) for corrosion protections [2]. The former targets at high temperature applications, e.g., in waste-to-energy boilers, while the latter aims at general applications to enhance the environmental sustainability, e.g., the fasteners and/or fittings of joining components in urban and offshore infrastructures. Via hot-corrosion of the In625 coatings in molten salts and electrochemical test of the SS431 coatings in artificial seawater, we found that the LC produced pore- and crack-free coatings are of great protective merits; however, the substrate dilutions, although mitigated by employing a dual-layer process, play key roles in corrosions at the near-interface regions, typically at the initial and/or last clad tracks, as does the heat affect zone (HAZ) of the LAS substrate. Additional surface enhancement, e.g., by cold-working, could further improve the corrosion resistance of the LC-produced alloys. These findings could have important consequences when developing protective coatings.
Material and Methods
20 mm thick high tensile LAS (AISI 4140) plates were used as the substrates for the LC processes of In625 and SS431. Both source materials are commercial powders, they were carried in pure argon and continuously fed through an annular coaxial nozzle during the LC process [see Fig. 1(b)]. The laser (~2.2 kW) was guided through an optical fiber and aligned through the center of the annular gap nozzle onto the workpiece at a beam size of 4.0 mm diameter.
During processing, the powder feeding and the scanning rate were set at 30 g/min and 1200 mm/min, respectively, which gave rise to a track width of ~1.8 mm. After completing a track, the longitudinal scanning was turned back at a transverse step-over distance of 2.0 mm. To stack up the coatings, the clad tracks in adjacent layers were parallelly aligned and collectively shifted by 1.0 mm along the transverse direction.
For the hot corrosion test, the In625/LAS heterostructures were buried in a powder mixture of Na2SO4 and MgSO4 (47:53 in mole ratio) contained in alumina crucibles and loaded into the heat zone together with sulphur powder at a low temperature zone in a tube furnace [3]. The hot zone was kept at 900 °C for up to 12 hours. The electrochemical tests were performed in a standard three-electrode corrosion cell using artificial seawater (i.e., 3.5 wt% NaCl solution) as the electrolyte. The corrosion protection was evaluated through the Tafel plots obtained by sweeping the applied potential and, meanwhile, recording the electrical current.
X-ray fluorescence (XRF), X-ray diffraction (XRD, Cu-Kα radiation), scanning electron microscopy (SEM), and electron backscatter diffraction (EBSD) were used to study the structural properties of the LC-produced coatings. Energy-dispersive X-ray (EDX) has also been employed to analyze the hot corrosion-induced surface changes.
Results and Discussion
The photographs presented in Figs. 2(a) and 2(b) were taken from the cross-section of the as-cladded In625/LAS heterostructure before and after a chemical etching in an acidic solution [i.e., 15 ml HCl (37%), 10 ml HNO3 (70%), and 10 ml CH3COOH (30%)], respectively. The former exhibits the In625-on-LAS heterostructure, free from pores and cracks. In contrast, the latter shows an apparent HAZ in the LAS substrate nearby the interface while the near-interface region of the In625 coating has been roughened and become brighter, especially in the first clad track [indicated by the arrow in Fig. 2(b)]. The inset profiles in Fig. 2(a) are the wt% compositions measured by XRF from the cross-section along the surface normal direction. They show that the substrate dilution has been apparently suppressed in the second In625 layer. Likewise, the insets in Fig. 2(b) are the surface height profiles, measured from the cross-section after the chemical etching across the first (black) and third (red) tracks. They provide evidence for more material loss from the HAZ than those from the base LAS while the In625 layer is intact upon the chemical etching except for the roughened near-interface regions.
Presented in Fig. 2(c) is a typical EBSD image, showing the phase distributions of γ-In625 and α-Fe across the interface of the as-cladded In625/LAS heterostructure. It shows a coexistence of γ-In625 and α-Fe at the interface formed due to substrate dilution. A SEM image taken on the cross-section of the first track after the chemical etching, i.e., the area indicated by the arrow in Fig. 2(b), is shown in Fig. 2(d). One sees that the lower region, i.e., closer to the interface, has been severely etched via pitting due to the larger substrate dilution over there. Both the chemical etching of the near-interface In625 and the more material loss from the HAZ at the first track than those at the others suggest that special care should be taken when cladding the first track and, from the corrosion protection point of view, this situation also occurs to the last clad tracks.
Figures 3(a) and 3(b) present the SEM images recorded from the corroded In625/LAS on the surface and cross-section, respectively. They reveal regular crystal structures on the surface and a continuous thin layer sandwiched by the surface layer and the base In625. This thin layer is more visible in the inset with an artificial contrast tuning. To identify the layer structures, EDX profiles were collected at the locations indicated in Fig. 3(b) and the results are presented in Fig. 3(c). They provide evidence for an immediate Cr2O3 on In625 covered by MgO. The XRD curve collected from the top surface is presented in Fig. 3(d) along with the standard patterns of MgO and In625. Their comparisons confirm the presence of crystalline MgO and Cr2O3. It is believed that the continuous Cr2O3 layer on the surface of In625 provides effective protection for the coating and hence the LAS workpiece under the severe corrosive conditions at high temperatures.
Figures 4(a)-4(d) present the cross-sectional morphologies of the crack-free SS431/LAS heterostructures. Typical equiaxed cellular structures are clearly seen for the SS431 coating in Fig. 4(c) with Cr-rich at the grain boundaries, which is remarkably different from those of In625 [2]. Diffusion of Cr from the SS431 cladding into the surface of the LAS substrate is revealed in Fig. 4(d), where the Cr composition measured by EDX at the root-like structures (~5.8 wt%) is much higher than their surroundings (~1.5 wt%).
Finally, the Tafel plots presented in Fig. 4(e) reveal that the corrosion current of the LC-produced SS431 is, indeed, remarkably reduced when compared with that of the LAS substrate. Also seen is that an additional surface enhancement, i.e., by robotic hammer peening [4, 5], can further improve the corrosion resistance of the SS431 coating. These observations shed light on further improvements in the LC process of SS431 for protective coatings.
Conclusion
LC has been recognized as a powerful technique in additive manufacturing, component repair, and portative coating. Its flexible switches in powder feedings among multiple sources, together with the fast heating and cooling characteristics make it an important tool in developing novel alloy materials with advanced functions. Through the case studies for LC processing of In625 and SS431 on LAS substrates, we found that special care should be taken when cladding the first and last tracks, where both the substrate dilution and the HAZ might cause severe problems under corrosive conditions because of their part exposures to the environment. The corrosion protection of the In625 coating in molten sulphate salts was realized via forming a continuous Cr2O3 thin layer at elevated temperatures. With the same LC parameters, the resultant microstructures of the SS431 coating are remarkably different from those of the In625 coatings. Diffusion of Cr from the coating material, i.e., SS431, into the surface of the LAS substrate has been observed. The corrosion resistance of the LAS substrate is indeed improved by the LC produced SS431 coating; however, our preliminary surface enhancement studies shed more light on further improvements of SS431 for protective coatings.
Acknowledgement
This work is supported by A*STAR RIE2020 advanced manufacturing and engineering (AME) programmatic grant through the structural metal alloys program (SMAP, Grant no. A18B1b0061). The authors would also like to thank Roy Lim and T. F. Wang from Dura-Metal (S) Pte. Ltd for their technical support.
References
[1] A. A. Siddiqui, A. K. Dubey, “Recent Trends in Laser Cladding and Surface Alloying” Opt. Laser Technol. Vol. 134, pp. 106619, 2021.
[2] H. Liu, et al., “Laser-cladding and interface evolutions of inconel 625 alloy on low alloy steel substrate upon heat and chemical treatments” Surf. Coat. Technol. Vol. 404, pp. 126606, 2020.
[3] H. Liu, et al., “Hot corrosion studies of a salt-coated Ni-based superalloy under flowing wet air and sulfur vapor ambient” Mater. Corros. Vol. 71, pp. 1608-1618, 2020.
[4] H. Liu, et al., “Robotic hammer peening-induced martensite in austenitic steels: Spatial distributions of plastic deformation and phase transformation” Procedia CIRP Vol. 87, pp. 297-30, 2020.
[5] H. Liu, et al., “Effects of Robotic Hammer Peening on Structural Properties of Ni-Based Single-Crystal Superalloy: Dislocation Slip Traces and Crystallographic Reorientations” Metall. Mat. Trans. A, Vol. 51, pp. 3180-3193, 2020.
Author: Hongfei Liu, Ivan Chee Kiang Tan, Yuefan Wei